ClearPoint Neuro Announces FDA Clearance of the SmartFrame Array™ Version 1.1


Stock Market News 2022-04-28

ClearPoint Neuro Announces FDA Clearance and First-in-Human Cases

Spark Biomedical Receives FDA Breakthrough Device Designation for

Stock Market News 2022-04-28

2022 Lumenis NuEra Tight RF Laser - Like a New
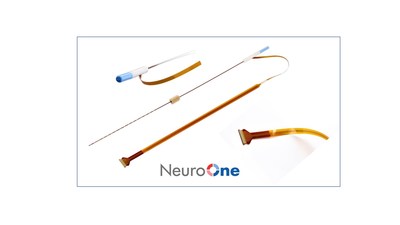
NeuroOne® Receives FDA 510(k) Clearance to Market its Evo® sEEG
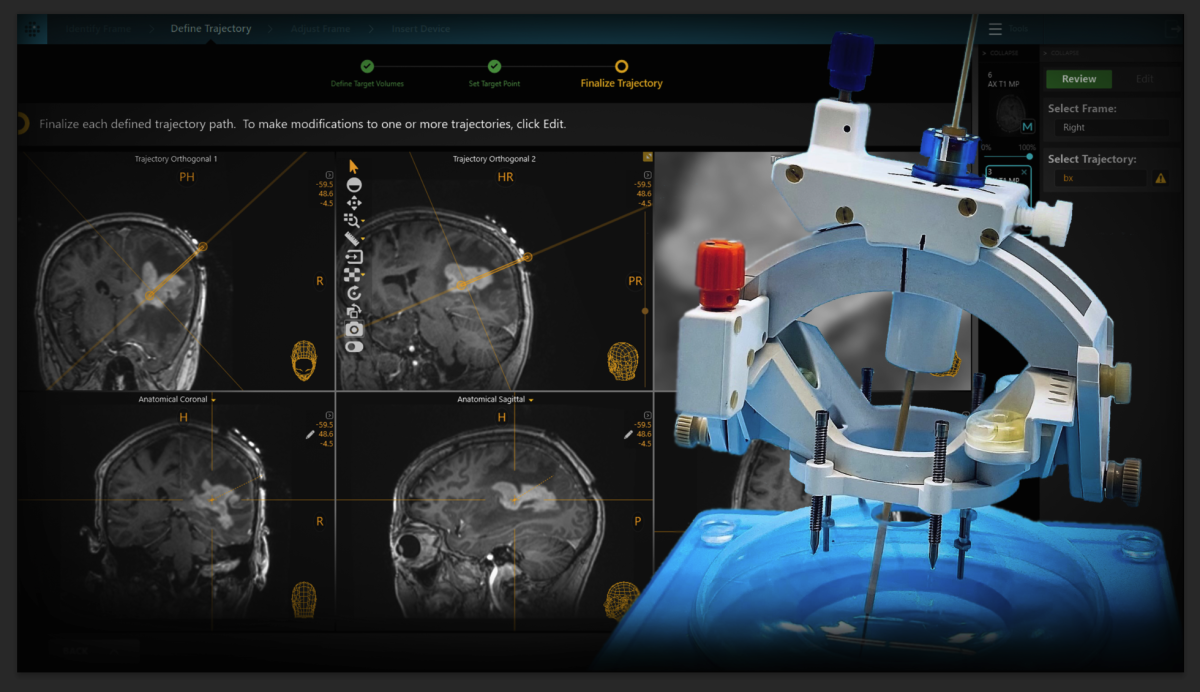
ClearPoint Neuro Announces FDA Clearance of the SmartFrame
Supply 308nm Excimer Laser System Dermatology KN-5000D Vitiligo

Clear-Com, VI-PNLB-12R-X4, V-Series IrisX panel: 12 Key, Rotary

2019 Lumenis NuEra Tight RF System

FDA Clears the SMART-C® ® Advanced X-ray Imaging System from
You may also like
Related products








