The FDA's rule change requiring providers to inform women about


Vital Signs: Digital Health Law Update, Fall 2023, Insights

Medical practitioners will have to notify patients about breast density in mammograms under new FDA regulations - CBS News

FDA to require mammogram providers to notify women about breast density to help detect breast cancer sooner

How UPMC Is Bringing AI into Patient Care - UPMC & Pitt Health

FDA Will Require Dense Breast Disclosure at Mammogram Clinics - The New York Times

Women Can Wait Longer Between Pap Tests, Doctor Reveal

Fresh from the biotech pipeline: record-breaking FDA approvals

The FDA Approves Cheaper, Over-the-Counter Hearing Aids
What Are Dense Breasts? FDA Requires Information In Mammogram Results

media./api/v1/images/stellar/prod/240116185

Page 7 – Women's Healthcare
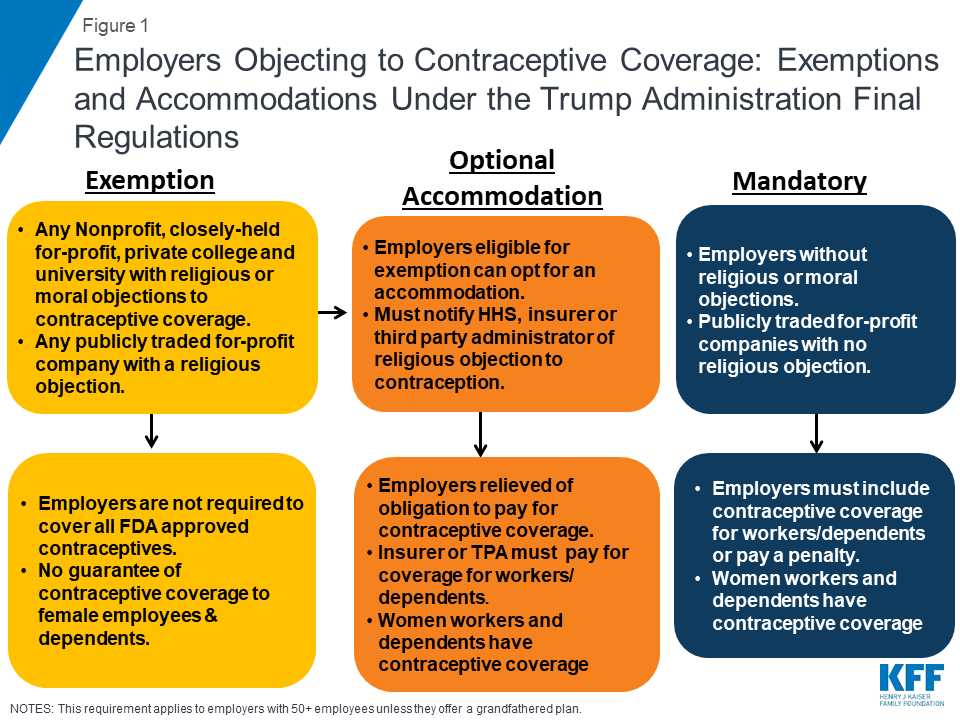
New Regulations Broadening Employer Exemptions to Contraceptive Coverage: Impact on Women
FDA says mammogram facilities must notify women if they have dense breast tissue - ABC News

Changes Ahead: Abortion Policy Proposals Affecting Reproductive Medicine, American Society for Reproductive Medicine









