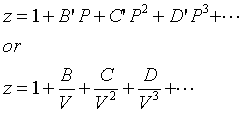At a high pressure, the compressibility factor (Z) of a real gas is us

At high P. P gt gt (n^(2)a)/(V^(2)) So ‘a’ can be neglected.

What is compressibility factor? What is its value for ideal gas

Real Gas

Compressibility factor (gases) - Citizendium
The compressibility factor for a real gas at high pressure is (a) 1+RT/pb (b) 1 (c) 1+pb/RT (d) 1-pb/RT - Sarthaks eConnect

Compressibility factor (z): real gases deviate from ideal behav-Turito

Objectives_template
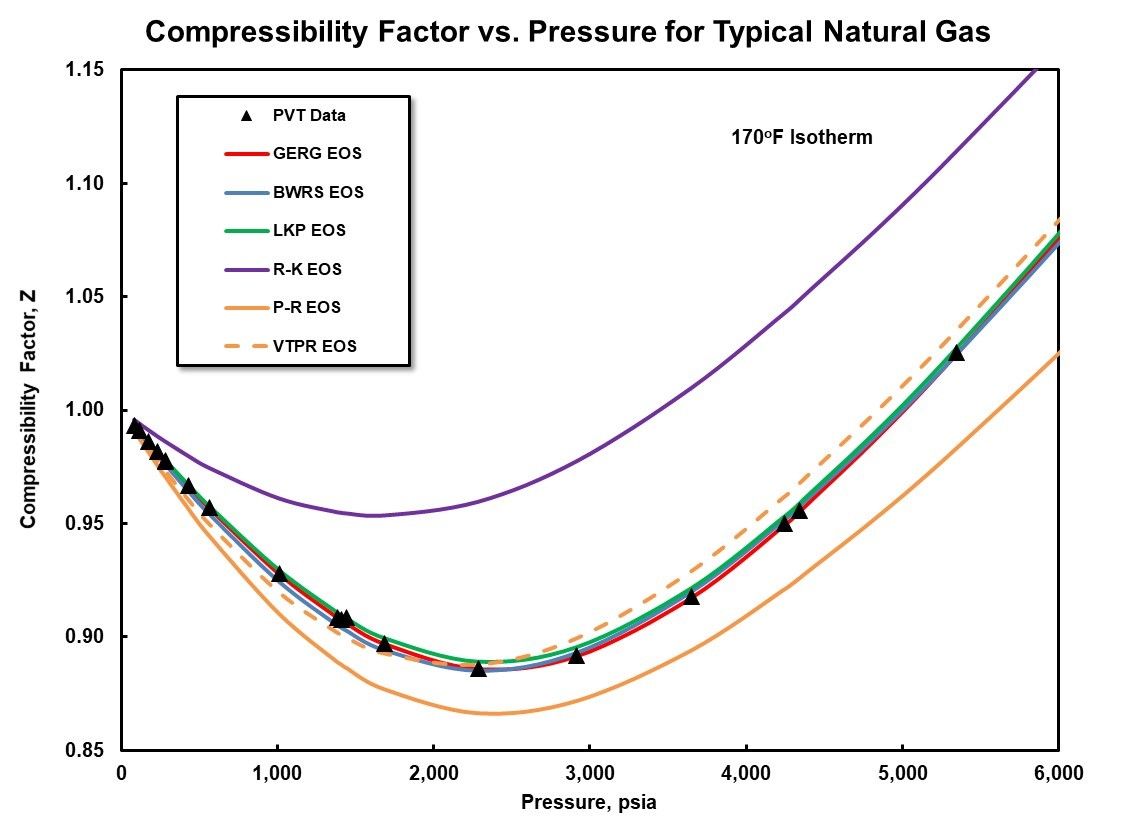
Compressor performance and thermodynamics

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT } (i) What is the value of Z an ideal gas?(ii) For real gas what will be

gas laws - How to find the temperature relationship between the isotherms in a compressibility factor (Z) vs pressure graph? - Chemistry Stack Exchange

JEE: Van der Waals Equation, Chemistry By Unacademy

Real gas z-factor, as attributed to Standing and Katz, 9 plotted as a

If assertion is true but reason is false.

At a constant pressure, what should be the percentage increase in the