Solved A diprotic base (B) has pKb values of 3.08 (pKb1) and

Equilibrium - Flip eBook Pages 51-100

Diprotic & Polyprotic Acids

SOLVED: A diprotic base B has pKa values of 3.54, 7.08, and 10.46. Which of the equalities is true when the pH is 6.92? [BH+] = [B] [BH] = [B] [BH+] = [
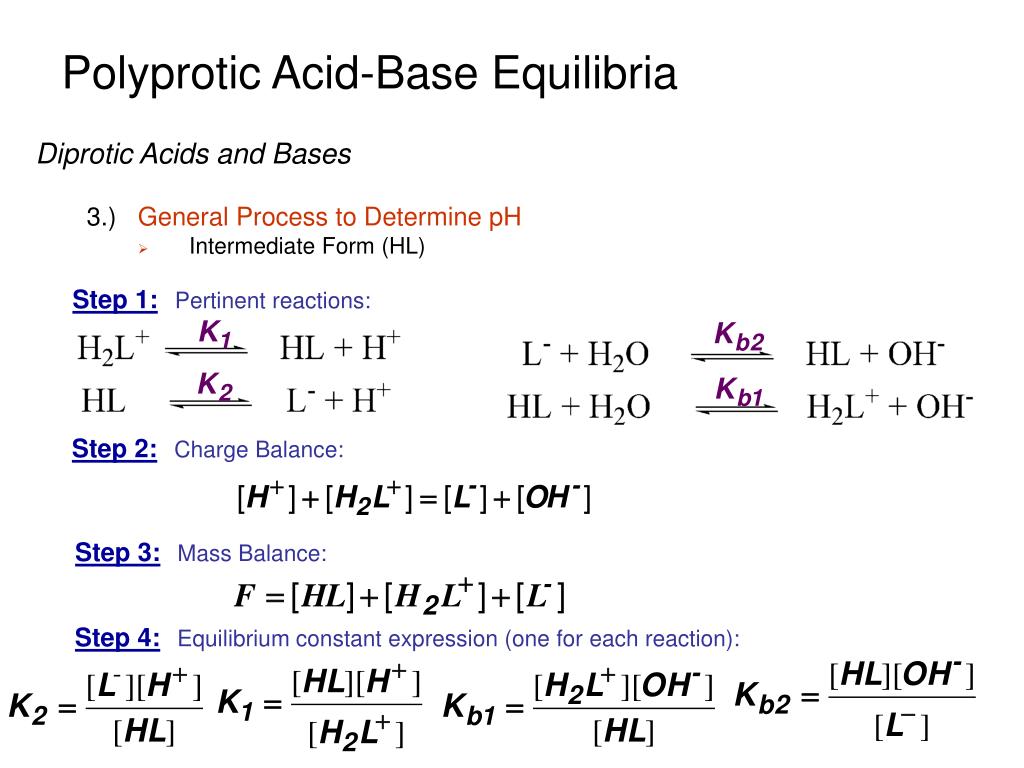
PPT - Polyprotic Acid-Base Equilibria PowerPoint Presentation, free download - ID:4342223
The pKb values for the dibasic base B are pKb1=2.10 and pKb2=7.54. Calculate the pH at each of the points

Acid-Base Equilibria 7.7 - Polyprotic Acids
Chapter 11: Acid-Base Titrations

An In-Depth Examination of Ionic Equilibria and Factors Affecting the Degree of Dissociation, PDF, Acid
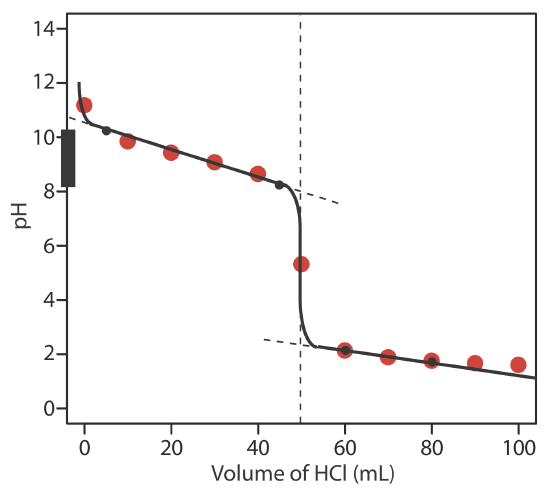
6.6: pH Calculations for Acid–Base Titrations - Chemistry LibreTexts

Diprotic Buffers - Video Tutorials & Practice Problems
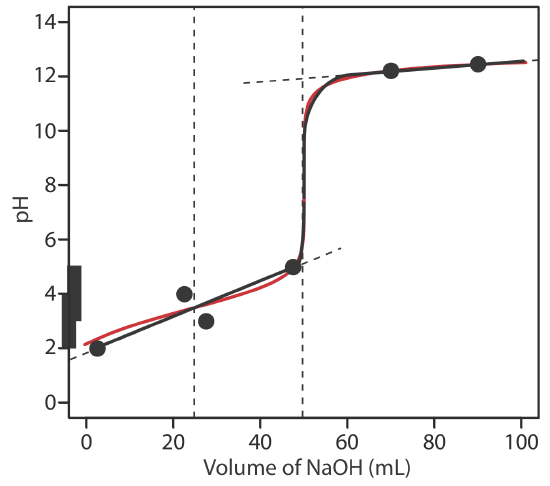
6.6: pH Calculations for Acid–Base Titrations - Chemistry LibreTexts









