At 300 K, 36 g of glucose present per litre in its solution has an osm

pi=CRT" (C = molar concentration)" (pi(1))/(pi(2))=(C(1))/(C(2))," "(4.98)/(1.52)=(36//180)/(C(2))" or "C(2)=(36)/(180)xx(1.52)/(4.98)="0.061 M"

Diabetes insipidus in infants and children - ScienceDirect

Effect of sodium administration on fluid balance and sodium balance in health and the perioperative setting. Extended summary with additional insights from the MIHMoSA and TOPMAST studies - ScienceDirect
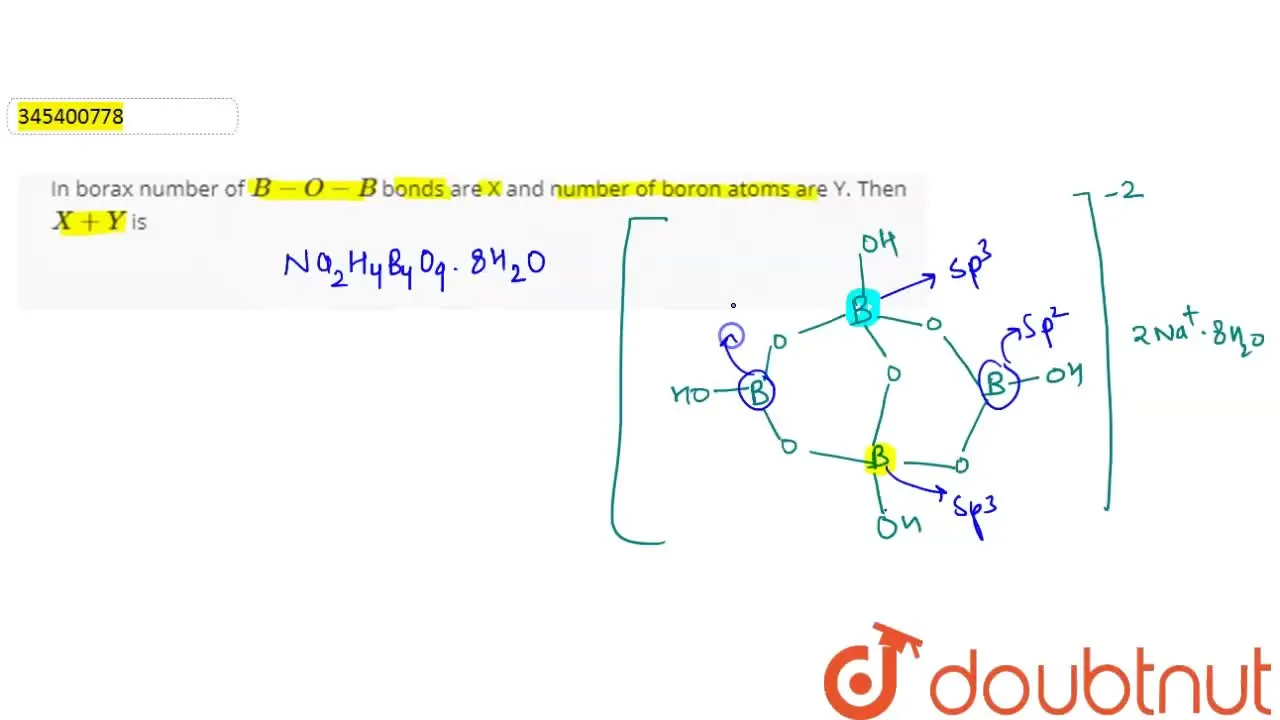
In borax number of B-O-B bonds are X and number of boron atoms are Y.
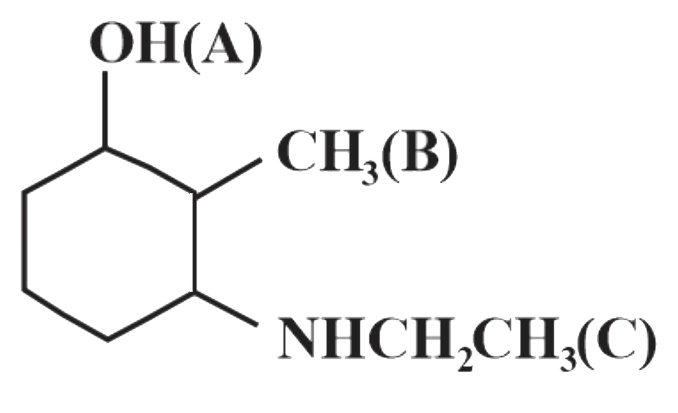
The current carrying ions are not necessarily discharged at the electr
PDF) Volume kinetics of glucose solutions given by intravenous infusion
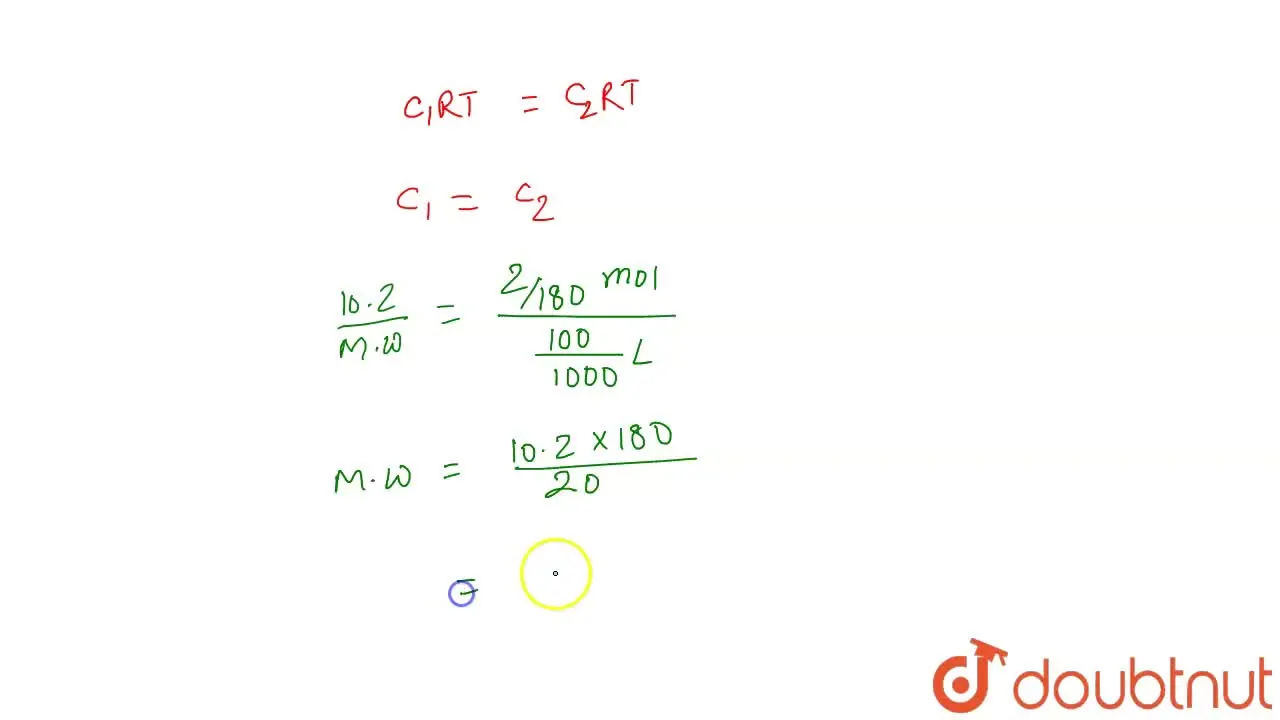
A solution containing 10.2 g of glycrine per litre is found to be isot

Lecture Notes: Chapter 1-Science and Measurements
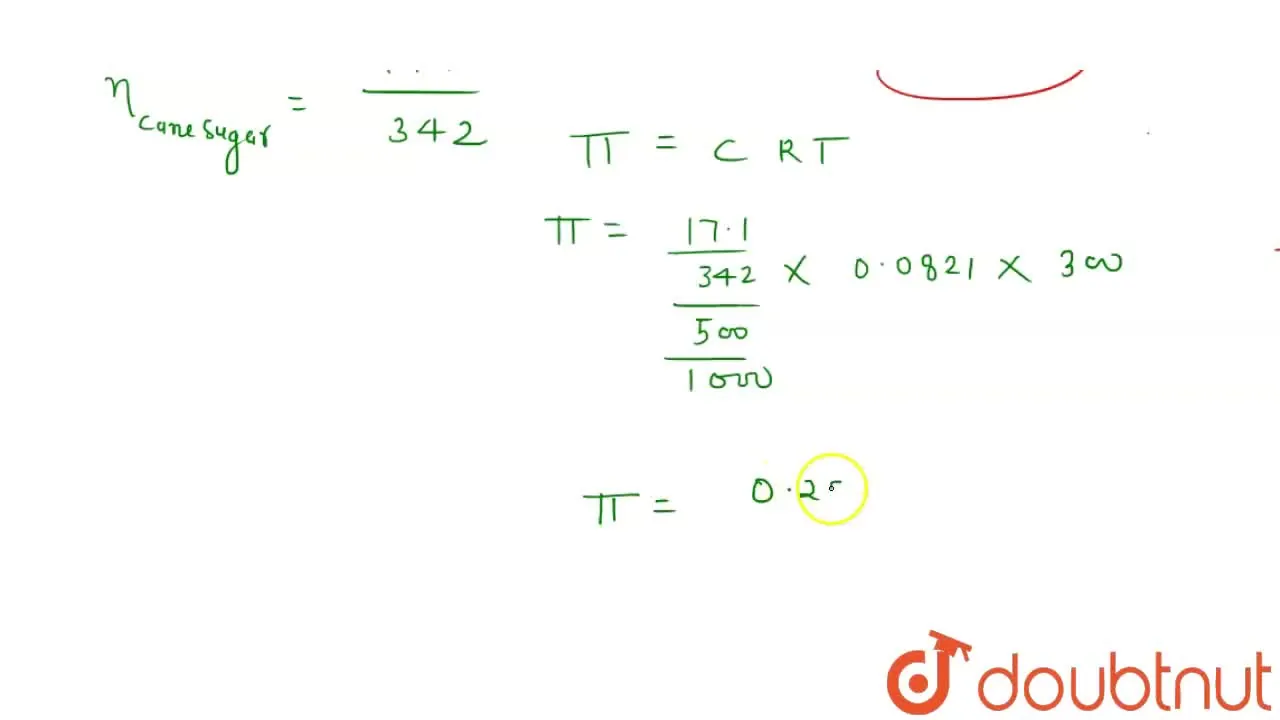
Calculate the osmotic pressure of a solution containing 17.1 g of cane

At 300 K, 36 gof glucose present in a litre of its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of the solution is 1.52 bars the same

16 Solutions

Unit 9: Critical care Analytes and Electrolytes & water balance

Based on solute - solvent interactions, arrange the following in order









