Solved An ideal gas initially at Pi, Vi, and T; is taken
Fick's laws of diffusion - Wikipedia
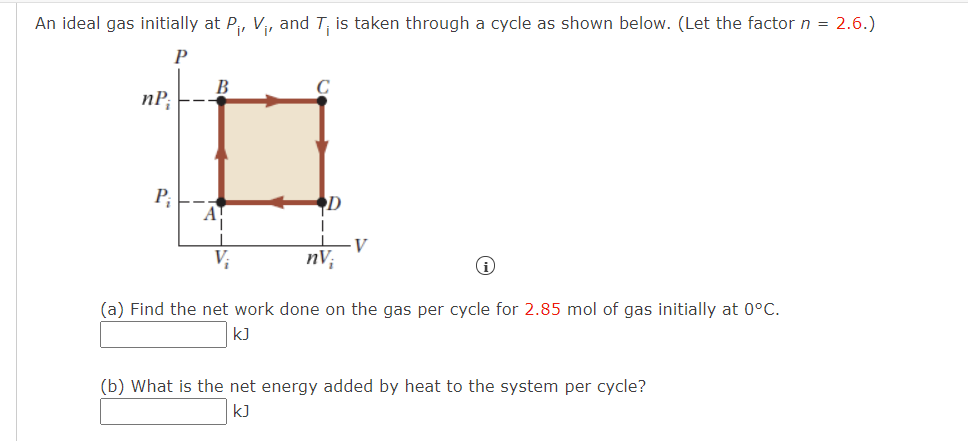
Solved An ideal gas initially at Pi, Vi, and T; is taken

Joule expansion - Wikipedia

An ideal gas initially P_i ,V_i , and T_i is taken through a cycle

An ideal gas is taken through the cycle `AtoBtoCtoA,` as shown in
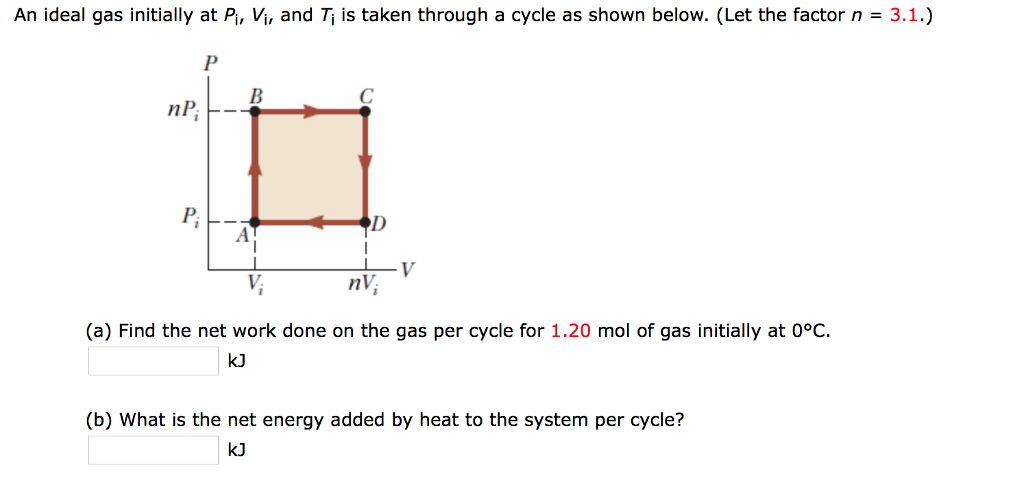
Solved An ideal gas initially at Pi, Vi, and T is taken

A 2.00-mol sample of a diatomic ideal gas expands slowly and
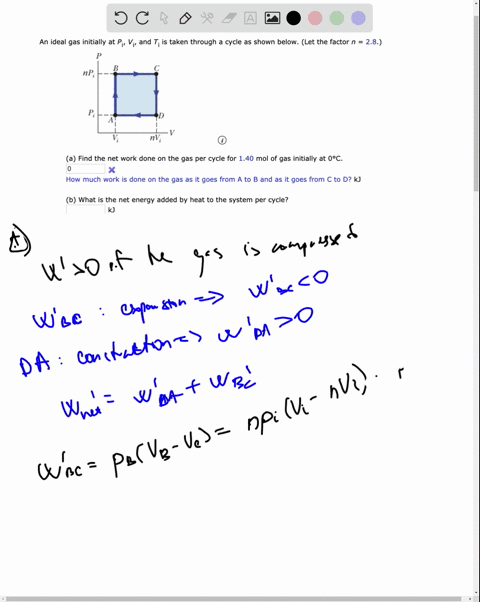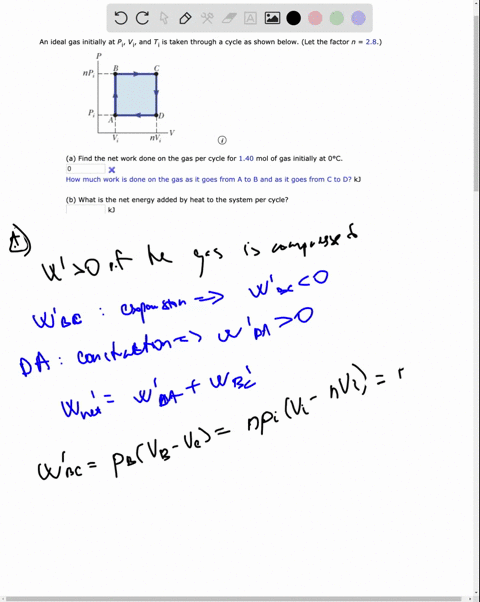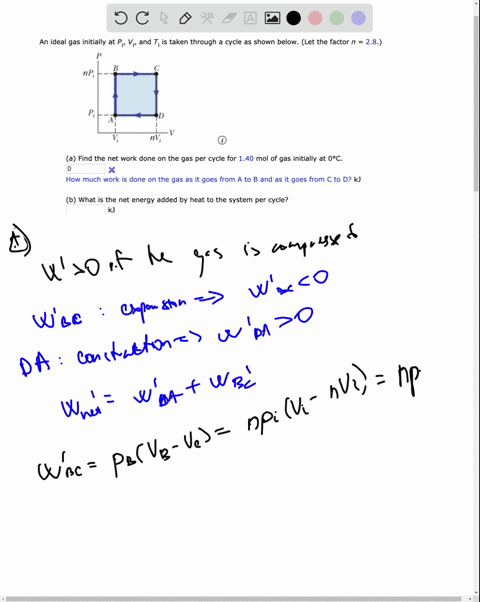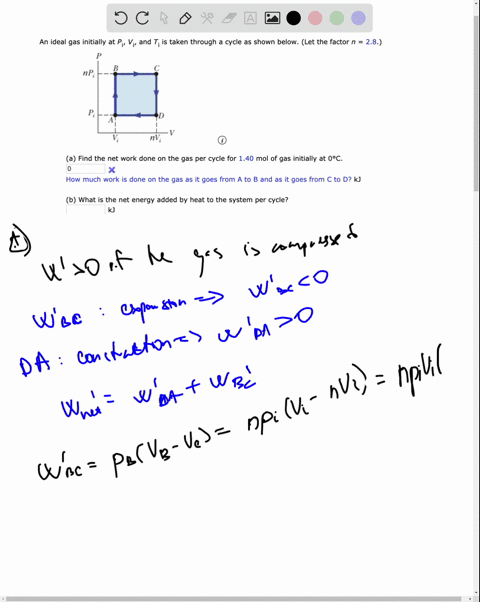
SOLVED: An ideal gas initially at Pi' Vi' and Ti is taken through
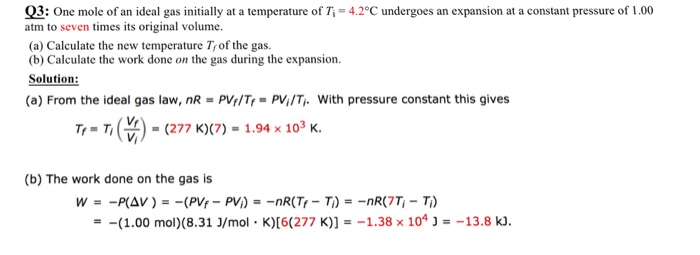
Solved For part b why is it 6 to multiple with 277 K ? I

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas
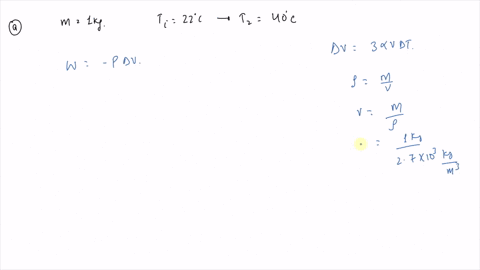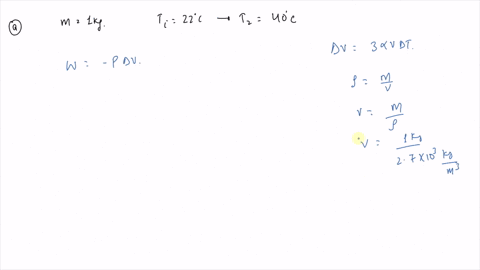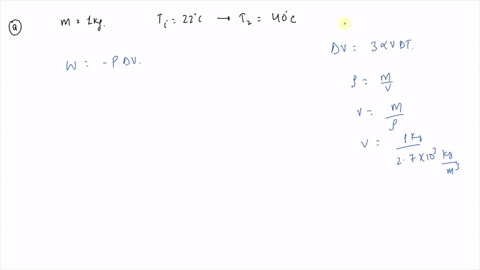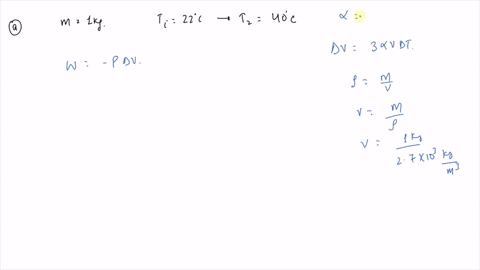
⏩SOLVED:An ideal gas initially at Pi, Vi, and Ti is taken through
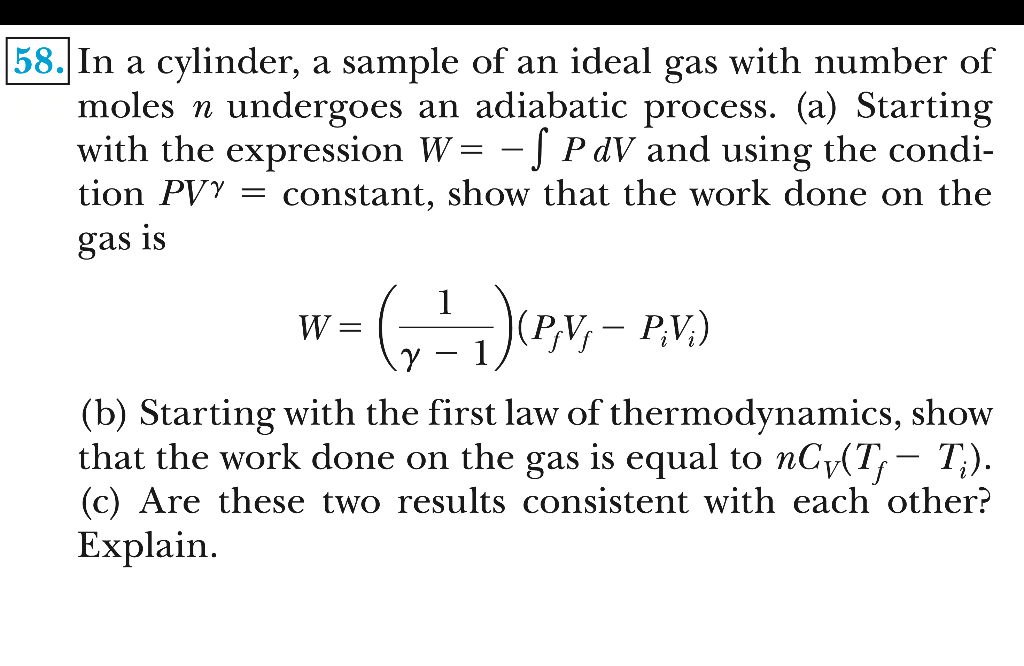
Solved In a cylinder, a sample of an ideal gas with number

Mean Free Path









