⏩SOLVED:Predict/Calculate A 35- g ice cube at 0.0^∘ C is added
VIDEO ANSWER: let the if is the temperature at thermal equilibrium. Then, by using love, congressional energy became right. He released way water but last year released Why Let me go! Less heat absolved way eyes is
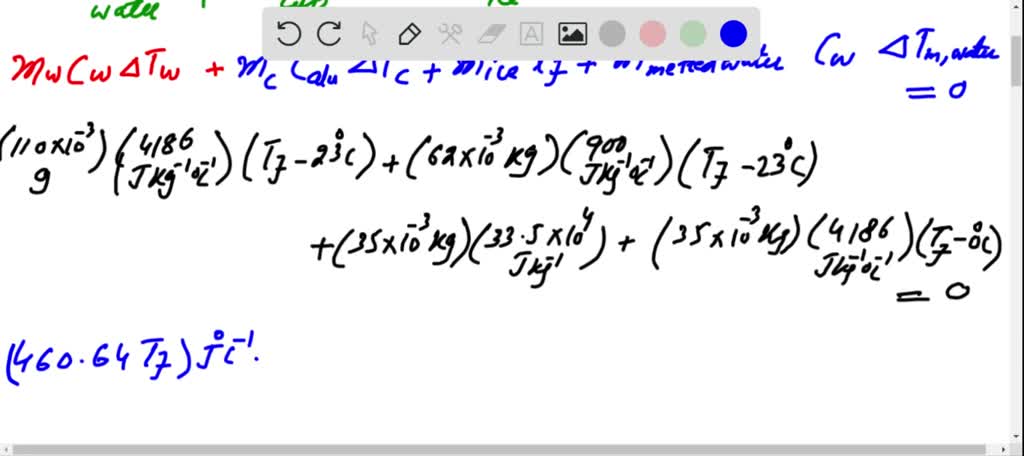
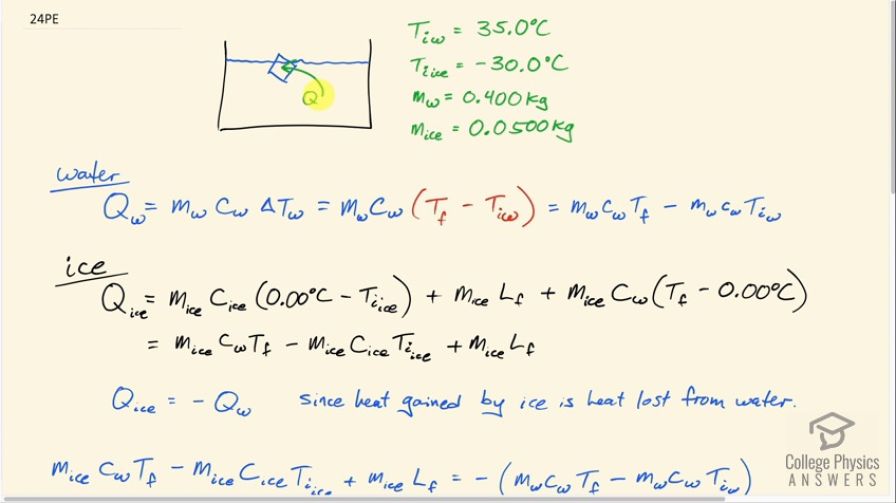
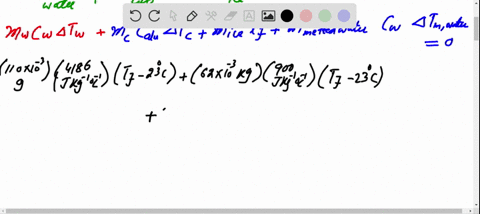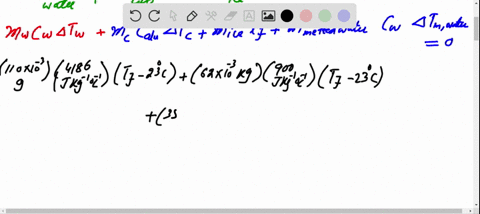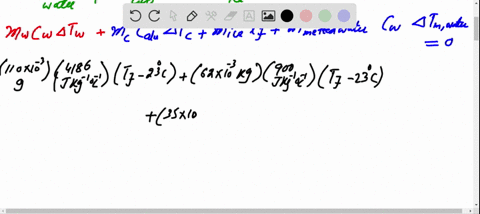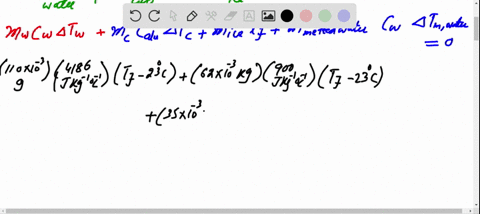
Predict/Calculate A 35- g ice cube at 0.0^∘ C is added to 110 g of water in a 62 -g aluminum cup. The cup and the water have an initial temperature of 23^∘ C (a) Find the equilibrium temperature of the cup and its contents. (b) Suppose the aluminum cup is replaced with one of equal mass made from silver. Is the equilibrium temperature with the silver cup greater than, less than, or the same as with the aluminum cup? Explain.
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.
OpenStax College Physics, Chapter 14, Problem 24 (Problems & Exercises)

SOLVED: A 35-g ice cube at 0.0 °C is added to 110 g of water in a 62-g aluminum cup. The cup and the water have an initial temperature of 23 °C. (
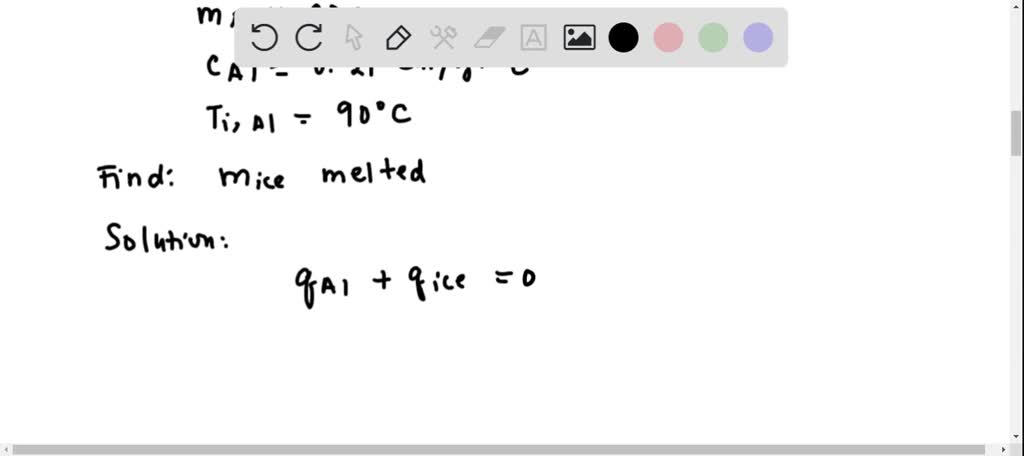
⏩SOLVED:A 20-g piece of aluminum (c=0.21 cal / g · ^∘ C) at 90 ^∘ C…
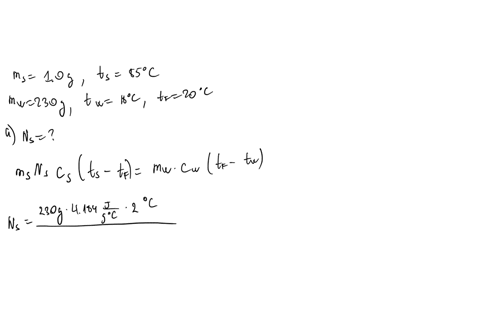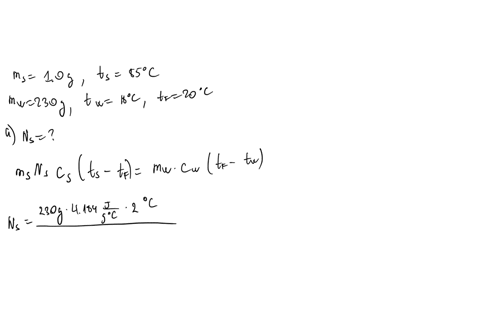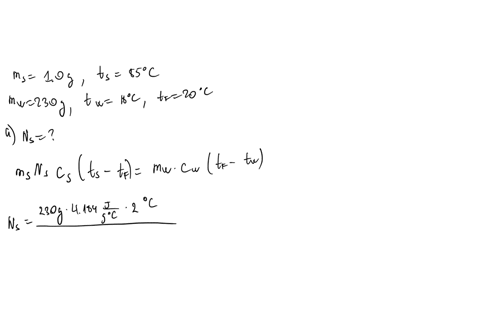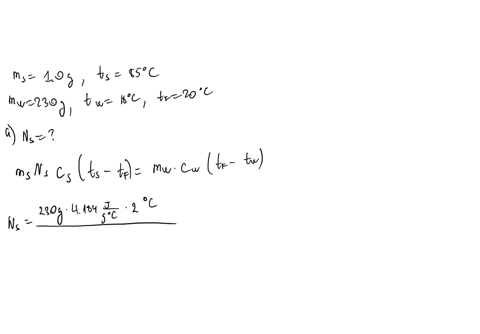
⏩SOLVED:∙Predict/Calculate Silver pellets with a mass of 1.0 g and a…
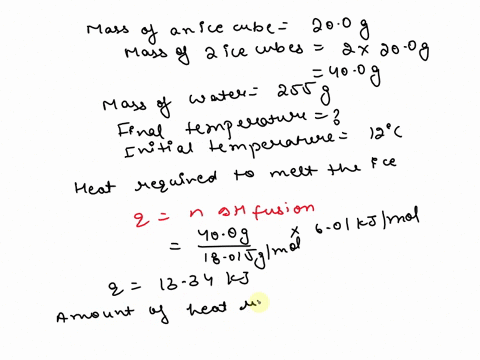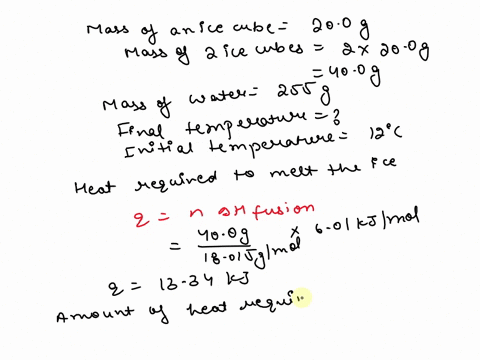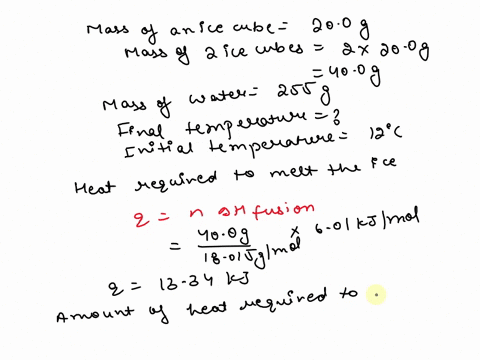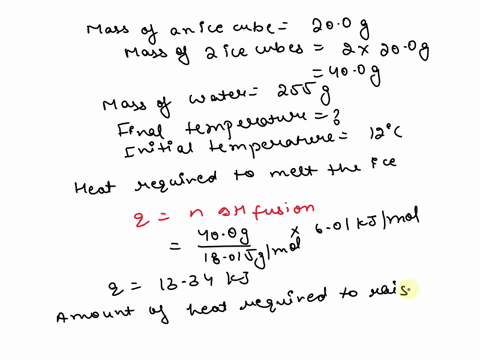
SOLVED: 15.0 g of ice cubes at 0.0°C are combined with 150 g of liquid water at 70.0°C in a coffee cup calorimeter. Calculate the final temperature reached, assuming no heat loss
⏩SOLVED:Predict/Calculate A 35- g ice cube at 0.0^∘ C is added to…

⏩SOLVED:20.58. A 0.0500-kg cube of ice at an initial temperature of…

Answered: A 10 g ice cube, initially at 0 ºC, is…
Solved] An ice cube at 0 degree Celsius weighing 100 g is dropped into 1 kg

⏩SOLVED:Referring to the previous problem, suppose the amount of…
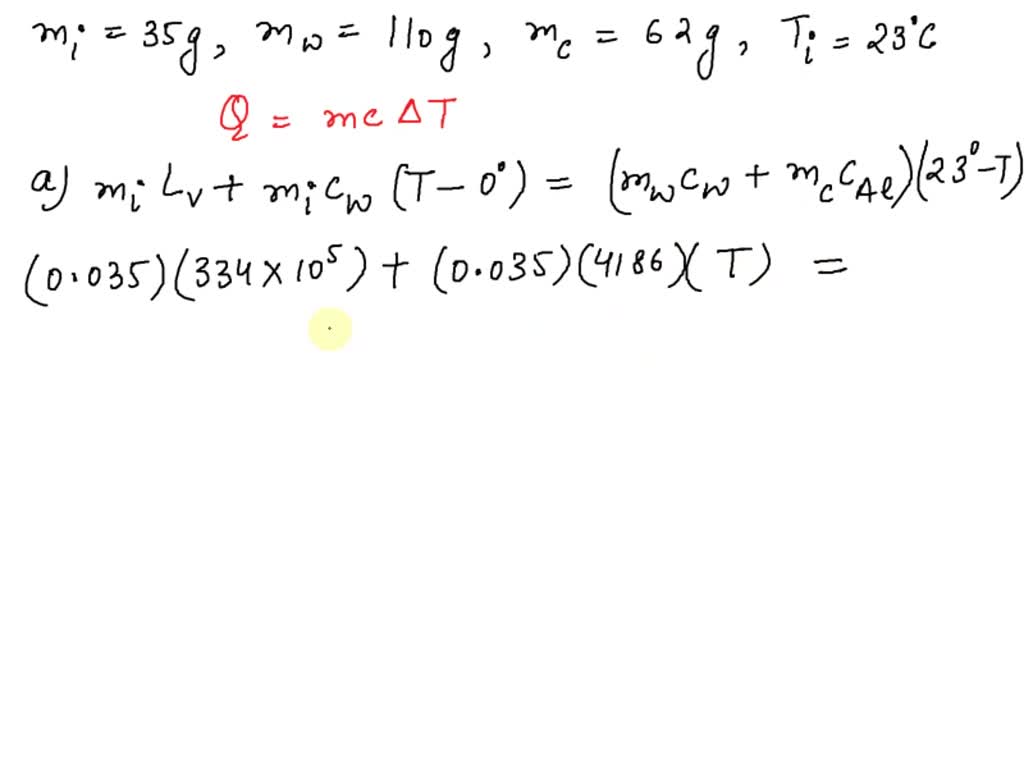
SOLVED: A 35-g ice cube at 0.0 °C is added to 110 g of water in a 62-g aluminum cup. The cup and the water have an initial temperature of 23 °C. (

⏩SOLVED:A 15.0 -kg block of ice at 0.0^∘ C melts to liquid water at…
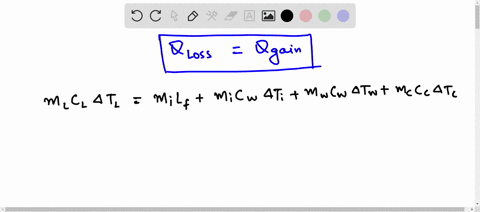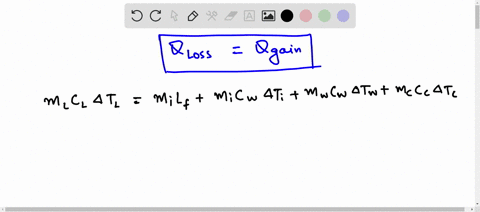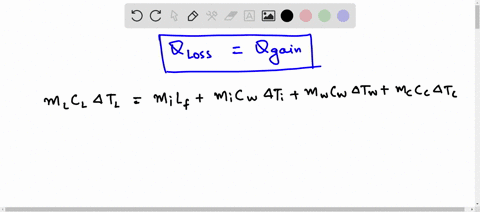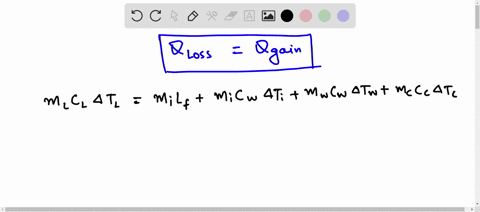
⏩SOLVED:A 48-g block of copper at -12^∘ C is added to 110 g of water…
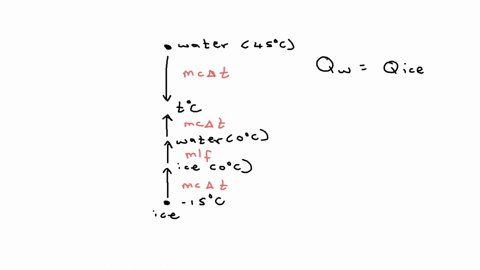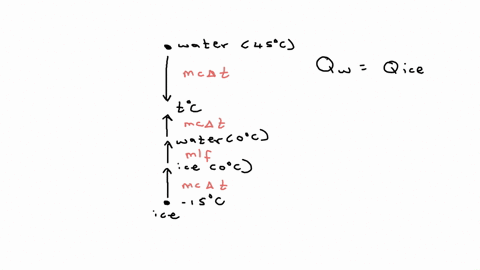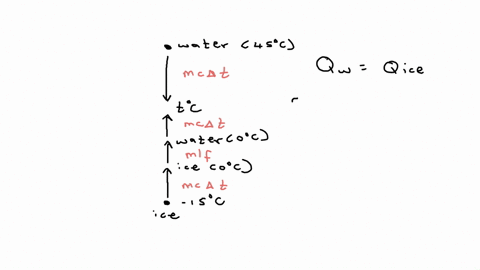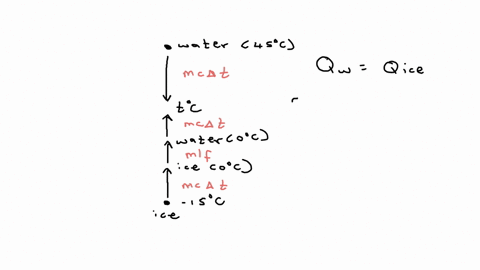
⏩SOLVED:20.58. A 0.0500-kg cube of ice at an initial temperature of…
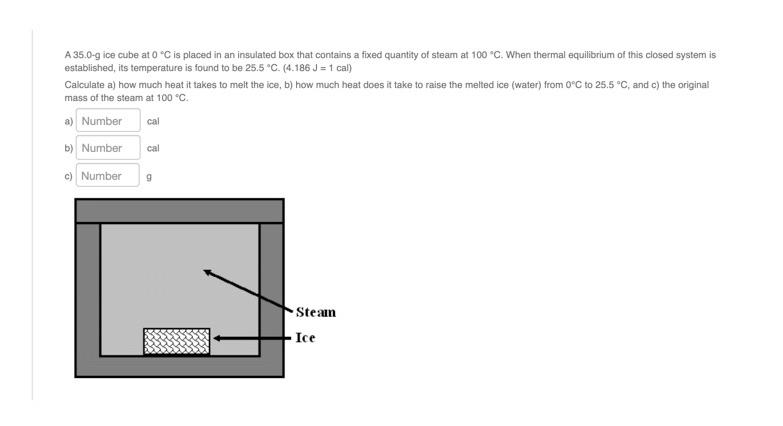
Solved A 35.0-g ice cube at 0 °C is placed in an insulated