Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2

Compressibility Factor Calculator - File Exchange - MATLAB Central

01 Gaseous State#### PDF, PDF, Gases
What does a compressibility factor >1 signify, apart from a deviation from the ideal gas behaviour? Is it more compressible? - Quora

Determine Compressibility of Gases

Solved The plot below shows how compressibility factor (Z)
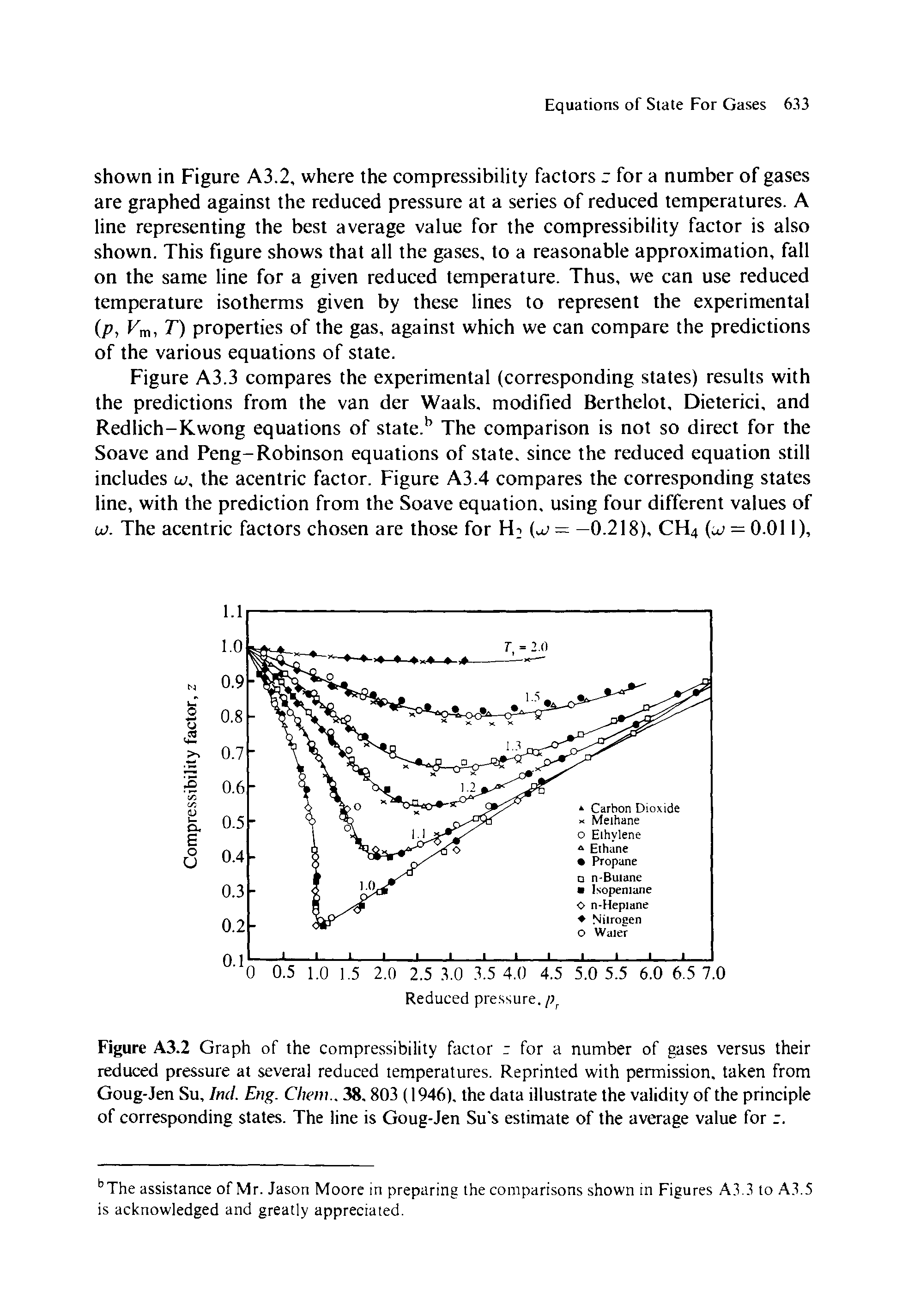
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure
What is the significance of the curve part in Z vs. P graph of compressibility of a gas? - Quora

Compressibility factor Z is plotted against pressure p for four different gases A , B , C & D. The correct order of critical temperature of the gasesA. A>B>C>DB. B>A>C>DC. D
Ideal Gas Equation and COMPRESSIBILITY Factor in 11 Minutes!
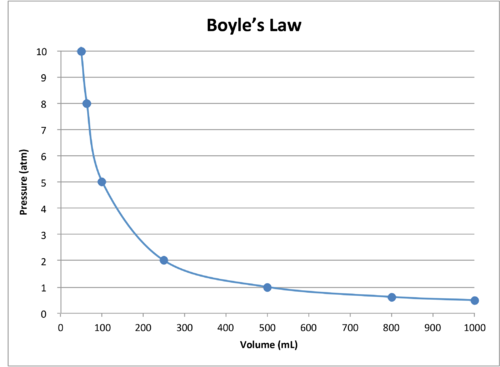
The Behavior of Gases Chemistry for Non-Majors