CORE-A End of Therapy (EoT) information : Clinical Outcomes in

PDF) A CORE approach to practice-based evidence: A brief history of the origins and applications of the CORE-OM and CORE System
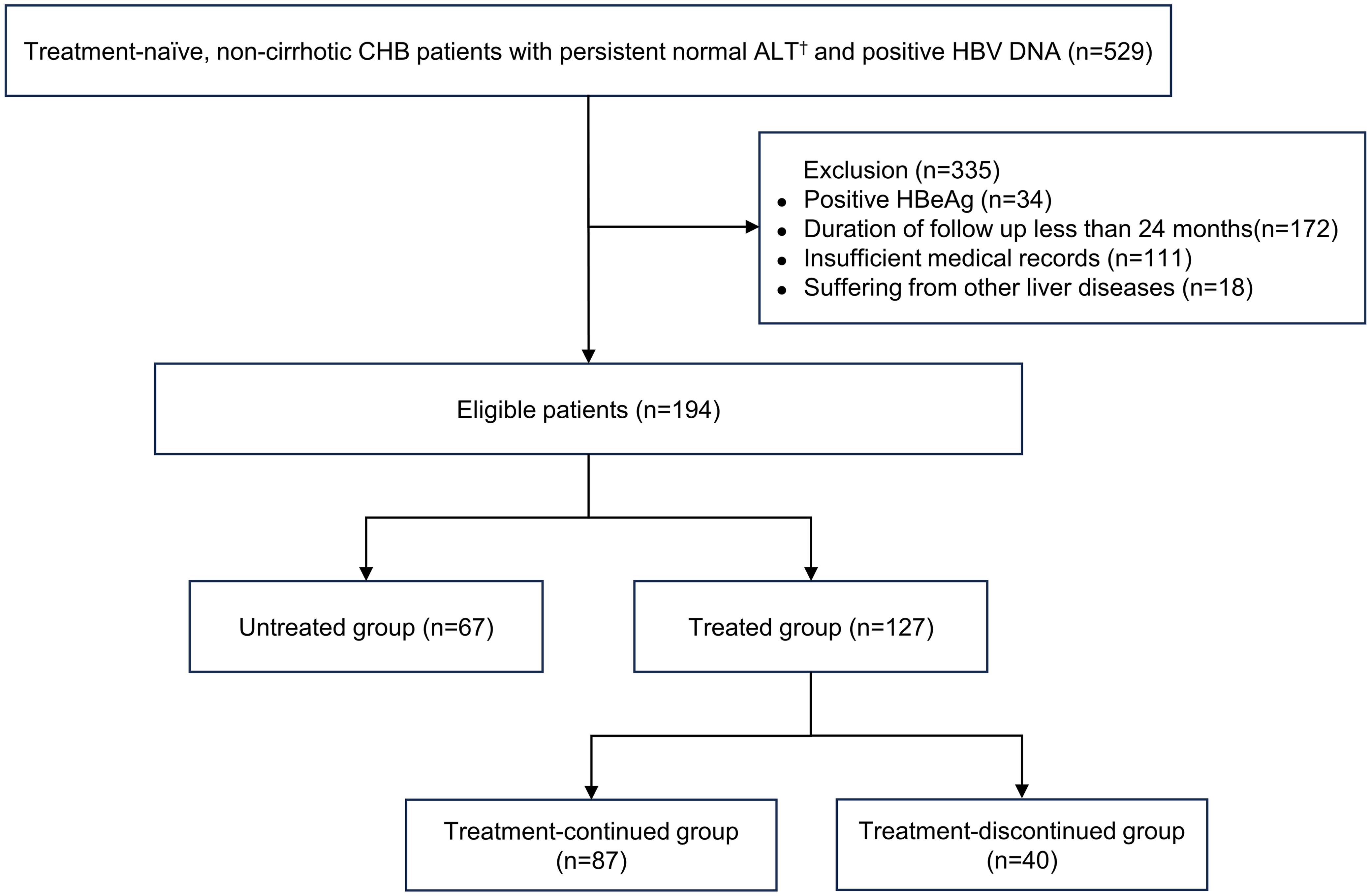
Antiviral Therapy Favors a Lower Risk of Liver Cirrhosis in HBeAg-negative Chronic Hepatitis B with Normal Alanine Transaminase and HBV DNA Positivity

Results. Time point 0: Baseline (assessment), 1: Psychiatric

Fillable PDF forms for CORE measures : Clinical Outcomes in Routine Evaluation (and CST)

Spanish CORE : Clinical Outcomes in Routine Evaluation (and CST)

Talking Therapists: Monitoring Change And Outcomes With The CORE System - WriteUpp Blog

POLARIX Trial
CORE-A End of Therapy (EoT) information : Clinical Outcomes in Routine Evaluation (and CST)

Study protocol of the FIRE-8 (AIO-KRK/YMO-0519) trial: a prospective, randomized, open-label, multicenter phase II trial investigating the efficacy of trifluridine/tipiracil plus panitumumab versus trifluridine/tipiracil plus bevacizumab as first-line

CORE (and CST: CORE System Trust) - Clinical Outcomes in Routine Evaluation (and CST)
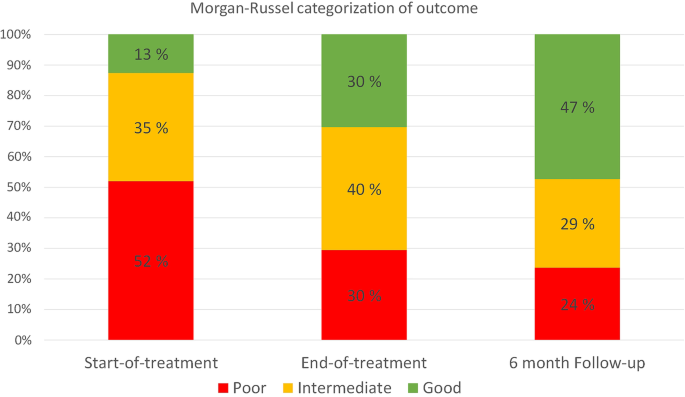
Multifamily therapy for adolescent eating disorders: a study of the change in eating disorder symptoms from start of treatment to follow-up, Journal of Eating Disorders

Patient profile using full CORE-OM (F), CORE Short Form A and B
Microsoft forms for CORE measures : Clinical Outcomes in Routine Evaluation (and CST)