Calculate the number of molecules of CO_2 present in 4.4 g of it.

Click here:point_up_2:to get an answer to your question :writing_hand:calculate the number of molecules of co2 present in 44 g of it
Click here👆to get an answer to your question ✍️ Calculate the number of molecules of CO-2 present in 4-4 g of it

No of molecules in 2.2 grams of `Co_(2)`
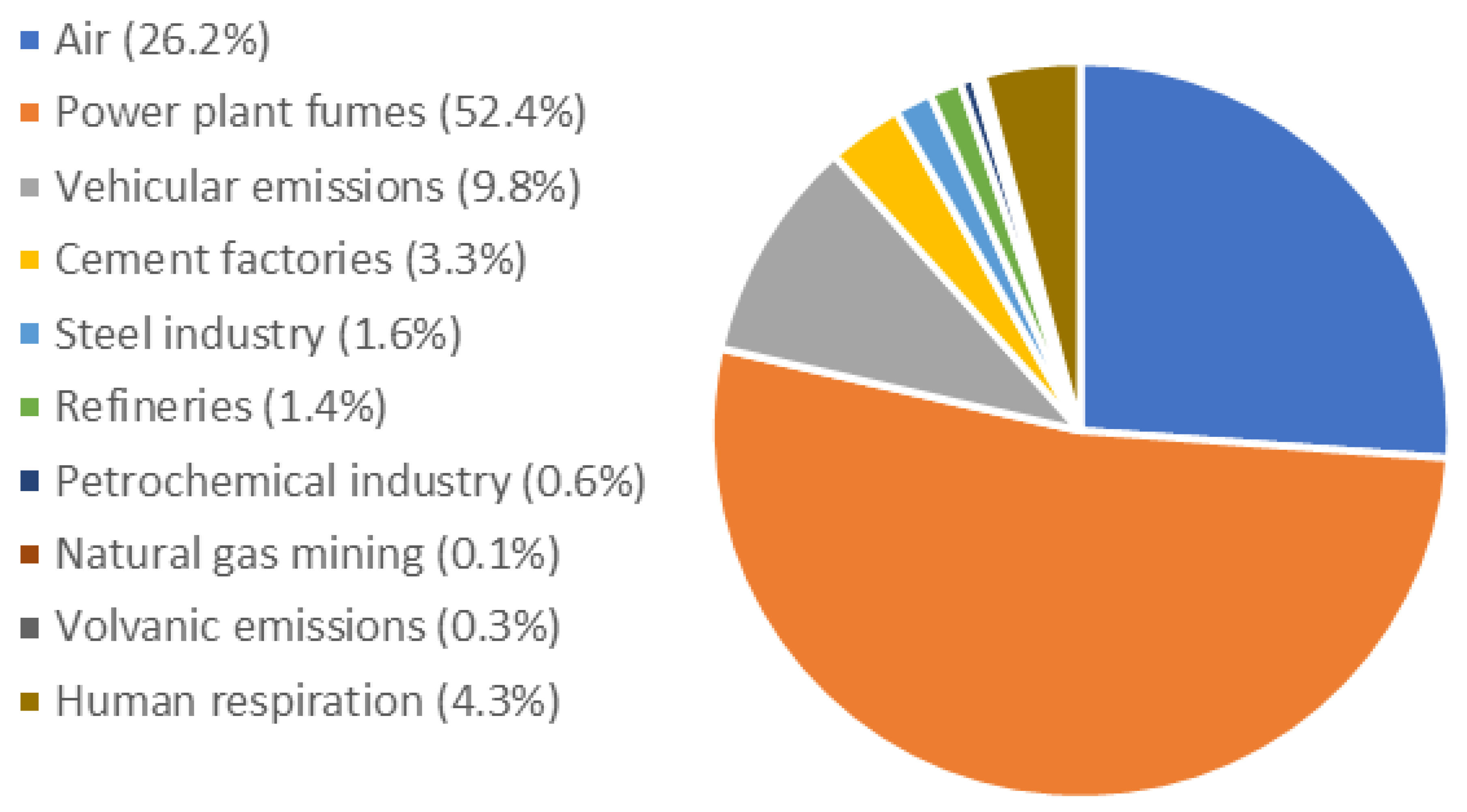
Atmosphere, Free Full-Text

A flask contains 4.4 gm of CO2 gas .calculate- 1. how many moles of CO2 gas are present in the sample? 2.
What is the mass of 2.24 litres of CO2 gas at STP? - Quora

Solved 7. How many molecules are present in 4.25 mol of
How many moles present in 5600 ml of CO2 at STP.

Gas Stoichiometry - Chemistry
2.24 l co2 gas at ntp find mass

Solved a. Calculate the number of atoms of each element

Volume of a gas at STP is `1.12xx10^(-7)` c c. Calculate the number of molecules in it

The number of oxygen atoms in 4.4 g of CO2 is(a) 1.2 × 10^23 (b) 6 × 10^22 c) 6 × 10^23

Plz Tell the 15th question Q 15 A flask contains 4 4 g of CO2 gas Calculate - Science - Atoms and Molecules - 12378457









