42g of N₂ react with excess of O₂ to produce NO. Amount of NO

Share your videos with friends, family, and the world

MOLE CONCEPT question bank(LEVEL-1) – Chemistry Tut0r
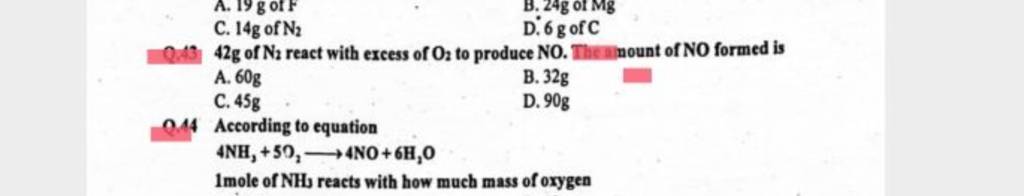
42 g of N2 react with excess of O2 to produce NO. Theninount of NO form..

Answered: Consider the balanced reaction of…

Mole Concept PDF, PDF, Mole (Unit)
7693-52-9, 4-Bromo-2-nitrophenol

Percent Yield Formula, How to Calculate Yield - Lesson

Solved If 10.0 moles of O2 are reacted with excess NO in the

Consider the reaction between NO(g) and O2(g) represented below. What is the balanced equation for this reaction and what is the limiting reactant?

Solved 18 18 Nitrogen monoxide, NO, and oxygen, 02, react to
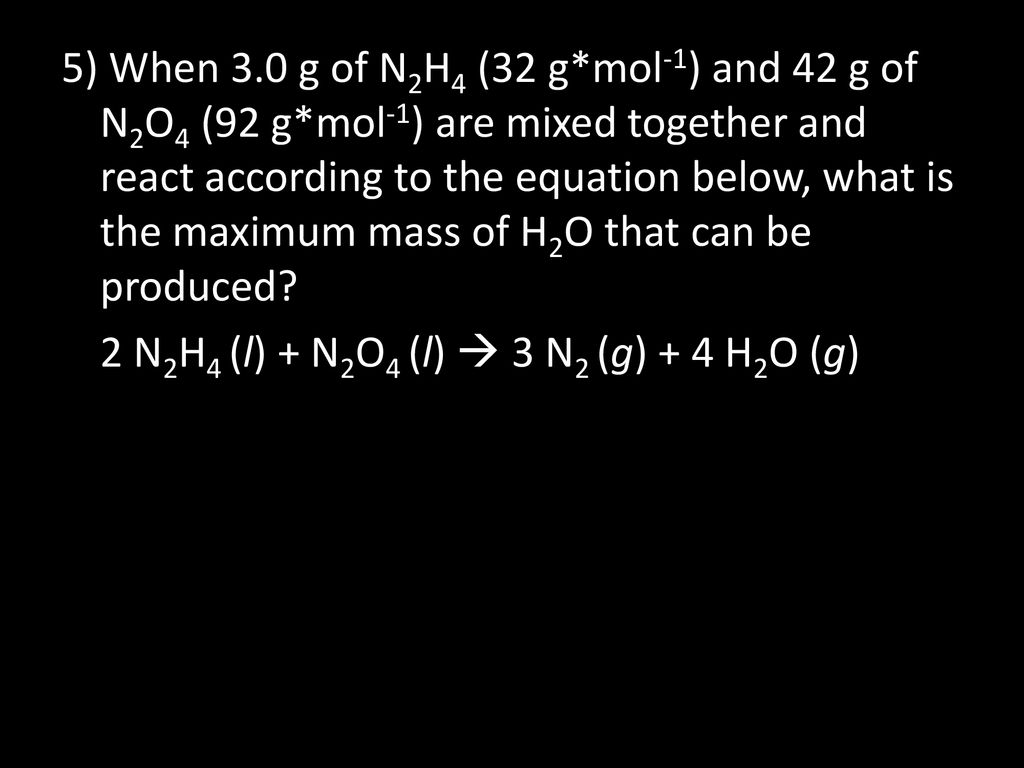
AP Chemistry Unit 2 Review: Choose your destiny - ppt download